Dobbins Lab for Advanced Materials Characterization
Dobbins Lab for Advanced Materials Characterization
Welcome to the Dobbins Lab for Advanced Materials Characterization
My research program focuses on gaining a fundamental understanding of the relationship between structure (at the atomic, nano-, and micro- levels) and dynamics (e.g. electron and phonon excitations, diffusion, and reaction kinetics) in composite materials. Properties of interest include surface adsorption, short range hopping dynamics, and long range diffusional transport. We use neutron and X-ray scattering as primary tools for gaining materials insights.
Tabbetha A. Dobbins, Ph.D.
Rowan University
College of Science & Mathematics
201 Mullica Hill Rd.
Glassboro, NJ 08028
dobbins@rowan.edu
Highlighted Research Activities
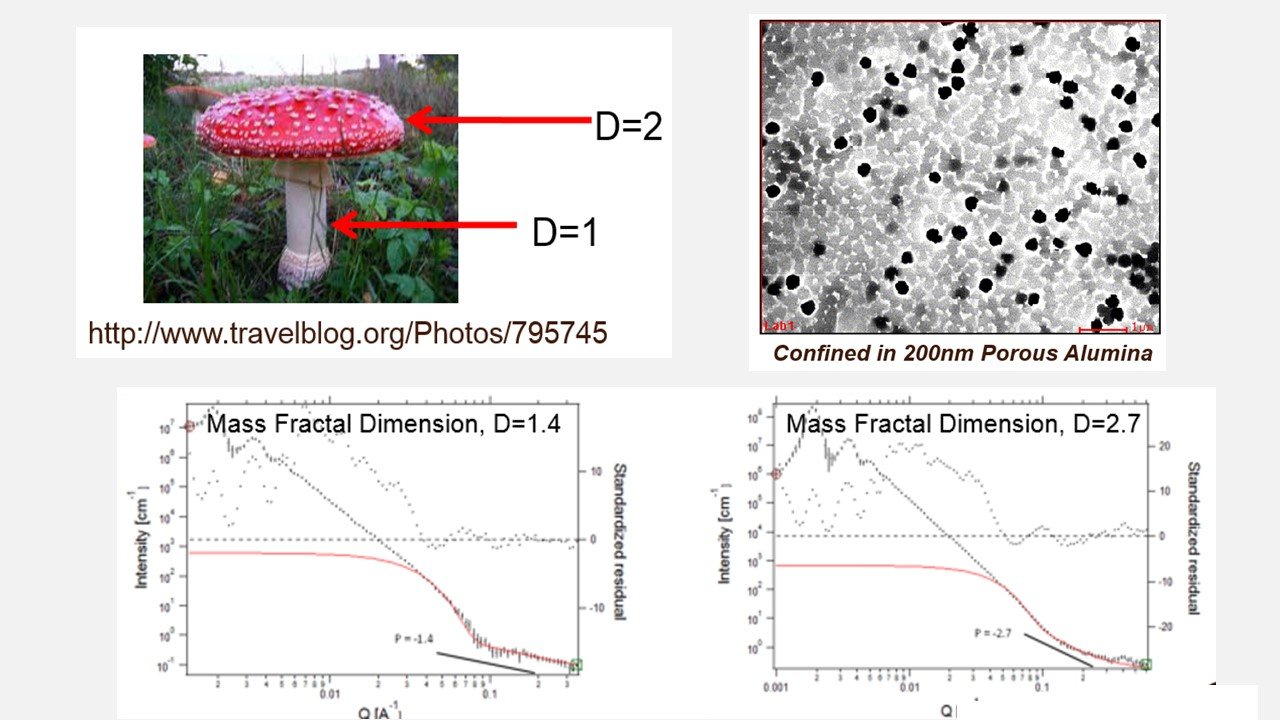
Hydrogen for Fuel Cells from NanoMushrooms Made of NaAlH4
Hydrides are used to deliver hydrogen for fuel cells for automotive applications. The shape (or morphology of those hydrides) determine the speed of hydrogen gas delivery (or kinetics). Nanostructuring is a key strategy in improving speed of hydrogen desorption reaction. Study of those nanostructures is made possible by synchrotron x-ray scattering performed at the Advanced Photon Source. X-rays are used to determine shape and size of nanoconfined hydrides by measuring mass fractal dimension (D). Initially, NaAlH4 powders are surface fractals. After infiltration into porous alumina, these powders are mass fractals.
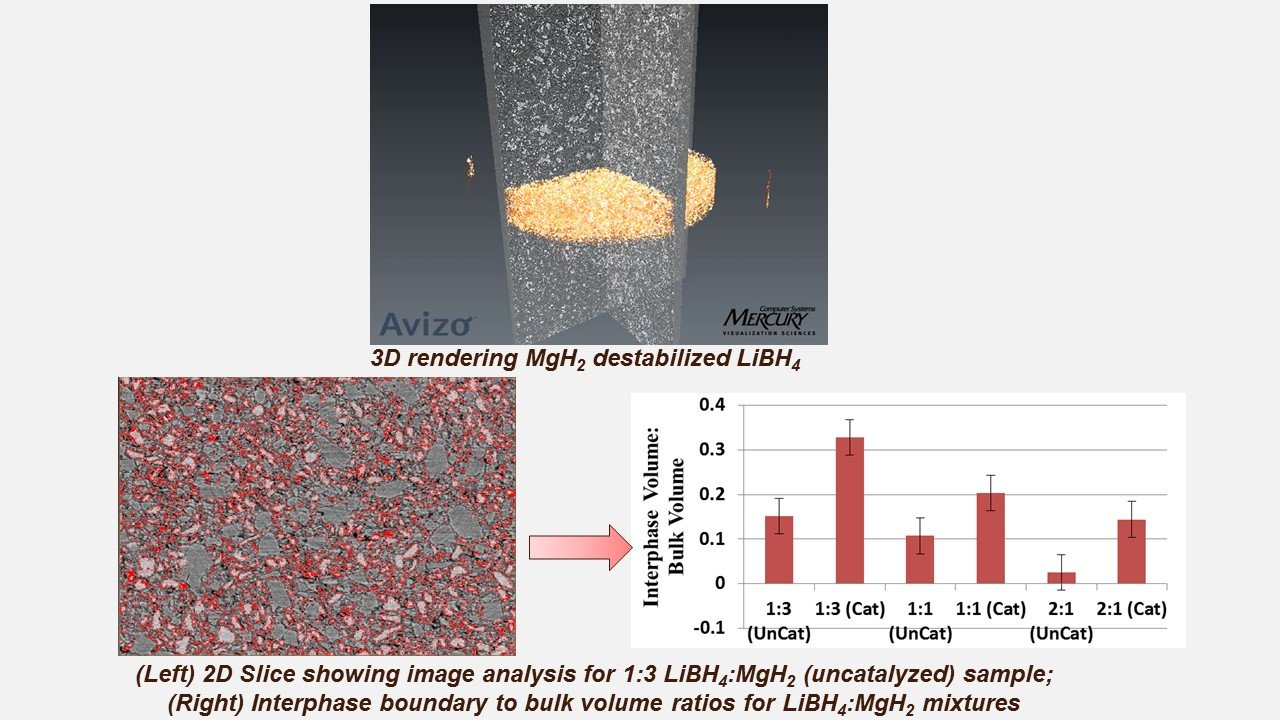
Kinetic limitations improved when Thermodynamic Destabilized Hydrides are doped with TiCl3
Kinetic limitations improved when Thermodynamic Destabilized Hydrides are doped with TiCl3
Thermodynamic destabilization occurs with formation of a stable alloy or compound comprised of the destabilizer atoms/ions and the H- coordinated cation on the hydride. Our 3D imaging work reveals that 4 mol % TiCl3 lowers kinetic barriers in MgH2-destabilized LiBH4 by increasing interphase volume (relative to bulk volume). In related work, we showed that TiN can serve as a destabilizer for NaAlH4.
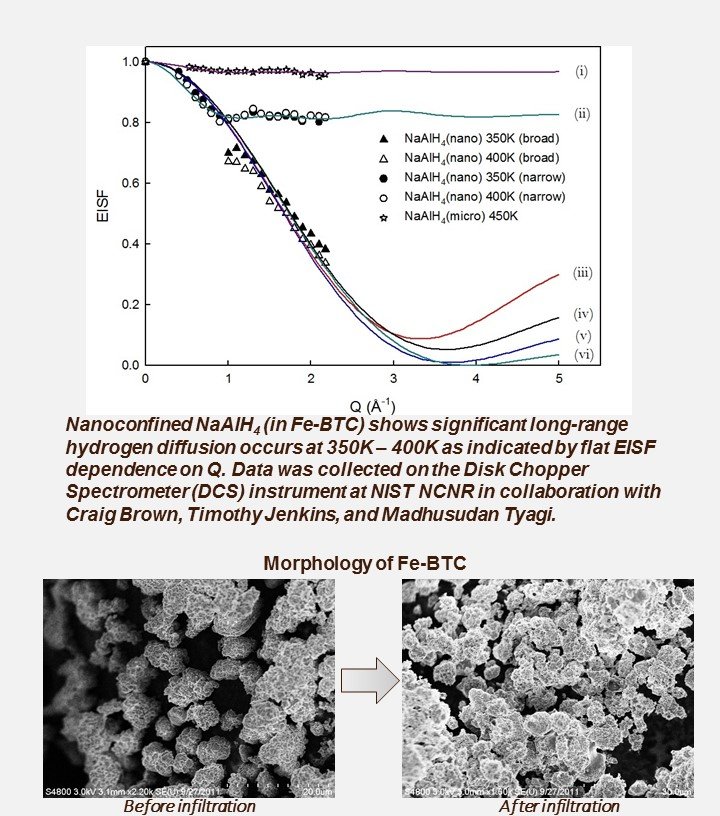
NaAlH4 nanoconfined in Fe-BTC MOF shows significant Hydrogen Mobility at 77oC
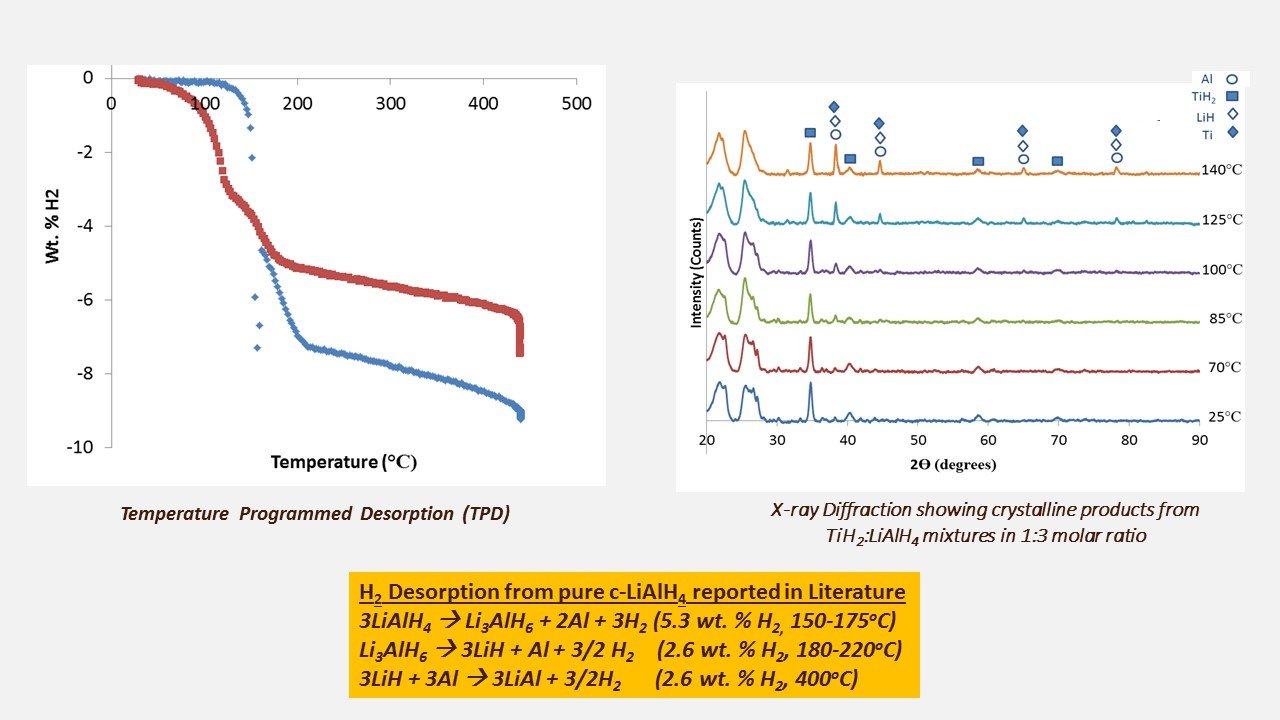
Solid State diffusion is enhanced in a-LiAlH4 when mixed with TiH2
Solid State diffusion is enhanced in a-LiAlH4 when mixed with TiH2
Mass transfer limitations inhibit H2 desorption from LiAlH4 at temperatures lower than melting. Recent work has shown that without melting, desorption does not occur for pure LiAlH4 heated repeatedly to just below melting at 150oC. However, after heating to above the melting temperature to 200oC, hydrogen is fully desorbed in 105s. Our work has examined enhancing atomic mobility with amorphous LiAlH4 (which is liquid-like on the molecular scale). We destabilize the a-LiAlH4 using TiH2 to yield Al (crystalline) and 4.8 wt % H2. Step 1 yields 3.0 wt % H2 at 77oC. Step 2 yields 1.8 wt. % H2 at 130oC.
Dobbins T., Ukpai W., Kinetics of H2 Desorption from TiN-Destabilized NaAlH4," ”, Materials Challenges in Energy Materials Challenges in Energy, Edited by Wicks G.G., Simon J., Zidan R. et al., 224, 99-106 (2011).
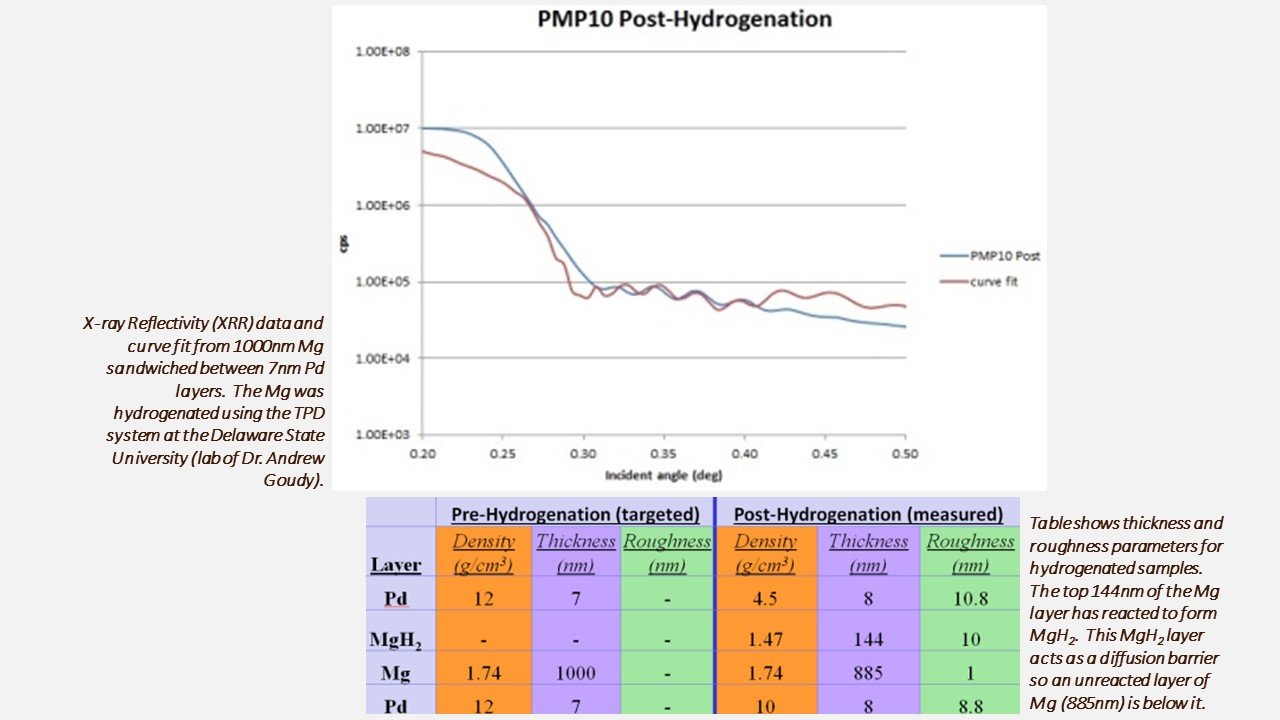
Diffusion limits H adatom adsorption into Mg to form MgH2
Diffusion limits H adatom adsorption into Mg to form MgH2
Our recently collected X-ray reflectivity film thickness data from Pd/Mg multilayers shows that a surface MgH2 film of 14.4nm forms and prohibits further hydrogenation. Since this layer is impermeable to H the hydrogenation of Mg requires a surface "activation“ (i.e. heating the Mg under vacuum conditions to remove it). Our future work will examine approaches to prevent the formation of this "native hydride" layer.