Kohout Lab
Kohout Lab
Welcome to the Kohout Lab
The Kohout lab studies how cells communicate. We focus on the interface between electrical and chemical signaling, thus integrating biophysics, molecular neuroscience, and cell biology. By understanding how these fundamental communication pathways work, we can better understand how and why they fail in disease states. Our current focus is on the voltage sensing phosphatase, a unique combination of a voltage sensor coupled to an enzyme, the only known voltage regulated enzyme.
Diversity, Equity and Inclusion Declaration
Racism is a pervasive and persistent problem in and out of STEM (1, 2, 3). I, Dr. Kohout, unequivocally condemn racism and stand together with all Black, Indigenous and People of Color (BIPOC), on and off campus. I am committed to creating a diverse environment and making my lab and my teaching safe, free and welcoming to all, regardless of color, gender identity, gender expression, sexual preference, ability status, age, physical appearance, body size, national origin, socioeconomic status, religion, spirituality or ethnicity. I also commit to identifying and dismantling any discriminatory policies and practices that may harm students, staff, faculty and our science.
I welcome people from a wide variety of backgrounds, not just because it is the right thing to do but because it makes our science, our teaching and our community stronger. I celebrate the diverse community different individuals cultivate and strives to create a space where all can develop to their full potential, bringing their full selves to the laboratory and classroom.
Research Overview
Signaling cascades are the basis for cell life. From single cells to multi-cell organisms, cells must respond to and communicate with their local environment. The higher the organism the more complicated that communication becomes with interconnected and interdependent signaling pathways. Failures in these communication pathways often lead to diseases when the cells can no longer compensate for the problems.
Communication pathways often start outside the cell, making the plasma membrane one of the most important sites for cell communication. The plasma membrane serves as a barrier separating the inside and outside of the cell. However, communication or signals still need to travel in and out of the cell meaning there has to be a mechanism for those signals to cross the barrier. Here’s where membrane proteins come in. They are responsible for transporting information into and out of the cell. This information flow allows the cell to “decide” on which critical cellular processes are needed, like migrating, dividing, differentiating, growing, and many others. Our lab currently focuses on how one protein, the voltage sensing phosphatase (VSP, Figure 1), bridges two fundamental communication pathways in the cell that stem from the plasma membrane.
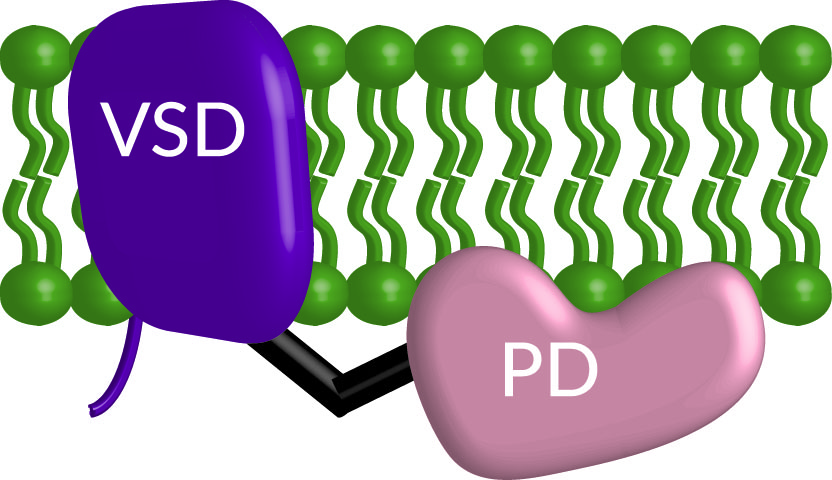
Figure 1. Cartoon of the voltage sensing phosphatase embeded in the membrane (green) with the voltage sensing domain (VSD, purple) and the phosphtase domain (PD, peach) connected by a linker (black).
First an introduction on the two types of communication pathways starting with the membrane potential. All the cells in our bodies are electrical in that they are more negative inside than outside. Neuronal action potentials are the classic example of electrical signaling but all our cells use the negative potential in one way or another. Muscles use them to trigger contractions. Pancreatic islet cells use them to specifically release insulin or glucagon. Immune cells use them to help activate an immune response. The list goes on. So the plasma membrane has a specialized set of membrane proteins that can respond to the changes in membrane potential, also called changes in voltage. These voltage sensitive proteins respond to the changes in membrane potential via a voltage sensing domain (VSD). The most common members of this family, the voltage-gated ion channels, use the VSD to open and close a pore domain, which allows for the passive movement of ions down their concentration gradients (Figure 2).
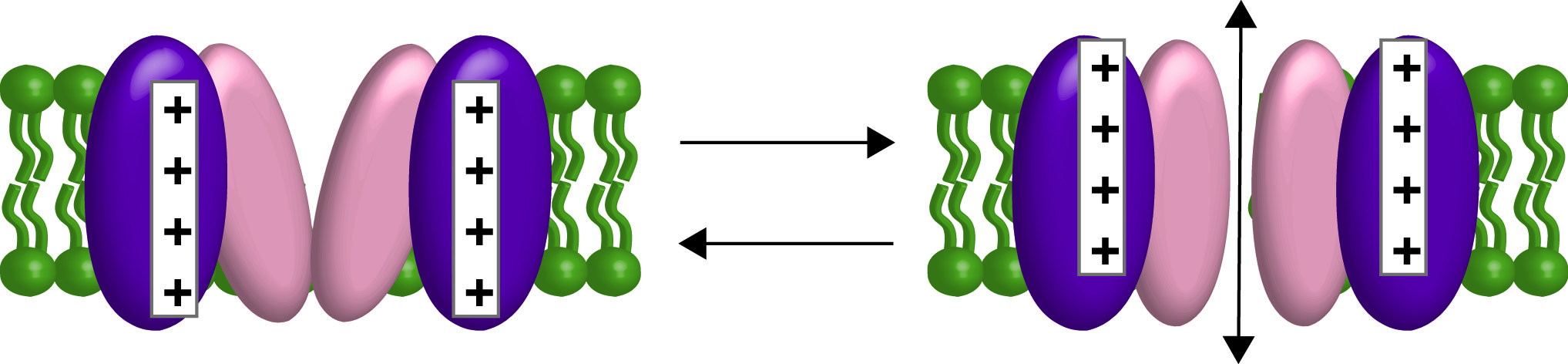
Figure 2. Cartoon of a voltage-gated channel opening and closing with the VSD in purple and the pore domain in peach. Ions will flow in and out of the cell depending on their electrochemical gradients.
The second signal we study are phosphatidylinositol phosphates (PIPs). PIPs serve in many different capacities within the cells. They act as a road map for protein sorting so proteins that need to function in the endosome are not accidentally targeted to the ER or lysosome. They also regulate many different cellular processes like growth, migration, cell death and they even help control channels. They can do all these things because they are lipids that populate the plasma membrane serving as a chemical signal that can be on or off by changing the number of phosphates on the inositol ring (Figure 3). Classically, control of the PIP signaling pathway has been attributed to soluble kinases and phosphatases that inter-convert the PIPs between forms that bind to distinct effectors. Since these effectors include ion channels and transporters, some of the fastest effects of PIP signaling are changes in membrane potential.
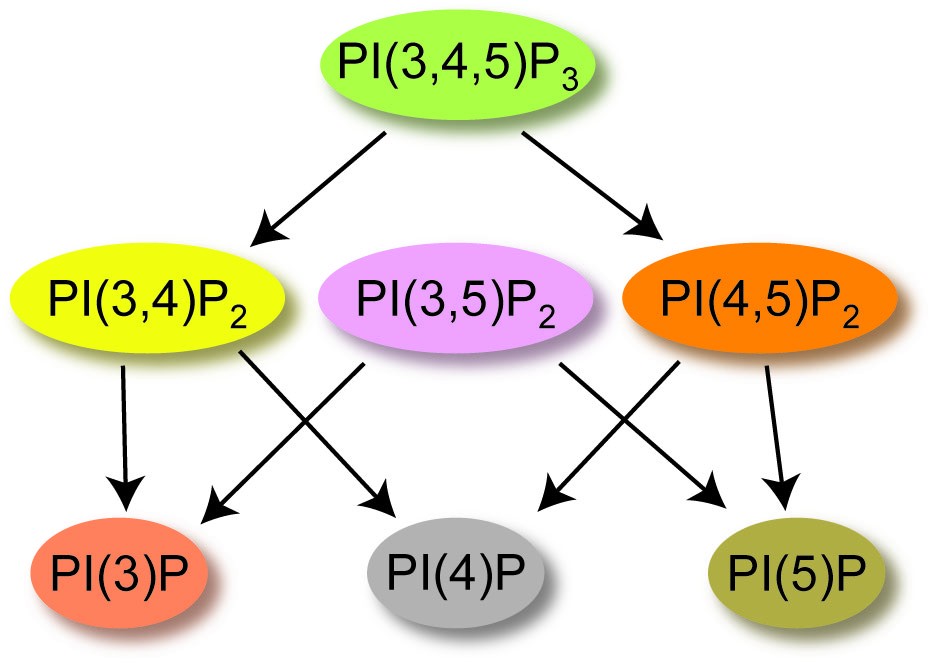
Figure 3. Cartoon of the dephosphorylation events for PIPs, starting with phosphatidylinosito-3,4,5-trisphosphate [PI(3,4,5)P3].
Back to VSP, it belongs to the same specialized family of voltage sensitive proteins as channels but is unique because it is the only member of that family that is not a channel. Instead of a pore, VSP has a phosphatase domain that dephosphorylates phosphates from PIP lipids (Figure 3). Thus, VSP responds to the electrical signals of the cell by altering the chemistry inside the cell. Because changes in membrane potential happen on a millisecond time scale, the activation of VSP will occur on a much faster time scale than the classic soluble phosphatases. Overall, VSP provides a fascinating feedback loop whose biophysical properties and physiological outcomes have yet to be determined.
To learn more, please explore our Research page!
To learn more about the members of the Kohout Lab, please explore our People and Lab Photo pages!