Research
Research
Kohout Lab Research
For decades, researchers have known about voltage-gated ion channels. When the voltage sensing phosphatase from Ciona intestinalis (Ci-VSP) was discovered in 2005 by Dr. Okamura and colleagues, it came as a shock to the field. Up to that point, all known voltage sensing proteins were ion channels. Some channels had associated enzymes, but their primary function was considered the channel opening and closing. Then in comes VSP, a protein that links a voltage sensing domain (VSD) directly to an enzyme in a way that had never been seen before. Once identified as a voltage sensing enzyme, VSP was found in many different organisms from frogs, fish, chickens all the way up to mice, rats and humans. The most unique feature of VSP is its voltage activation. Since the membrane potential can change on a millisecond time scale, that means VSP is also activated on a millisecond time scale, a much faster time scale than conventional phosphatases that activate in the seconds to minutes time scale. VSP’s discovery showed that an entire time scale of activation has been missing from investigations into PIP signaling.
While VSP is a member of the voltage sensing family of proteins, it is also a member of the phosphatase family of proteins. Specifically, the phosphatase domain from VSP is quite similar to the tumor suppressor, PTEN, sharing 44% identity between the two catalytic domains. PTEN is highly mutated in many different types of cancers and the similarity between PTEN and VSP could indicate a role for VSP in cancer progression. While VSP shares this high identity with PTEN, it is able to catalyze more reactions than PTEN because it can work as both a 3-phosphatase and 5-phosphatase while PTEN is only a 3-phosphatase (Figure 1). Specifically, VSP catalyzes the dephosphorylation of phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3] into phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] or into phosphatidylinositol-3,4-bisphosphate [PI(3,4)P2]. VSP subsequently dephosphorylates either [PI(4,5)P2] or [PI(3,4)P2] down to PI(4)P. Each of these PIPs are known to modulate important functions within the cell, from controlling growth and migration to regulating exocytosis and endocytosis. Understanding when cells require voltage control over their PIP concentrations is an open question in the field!
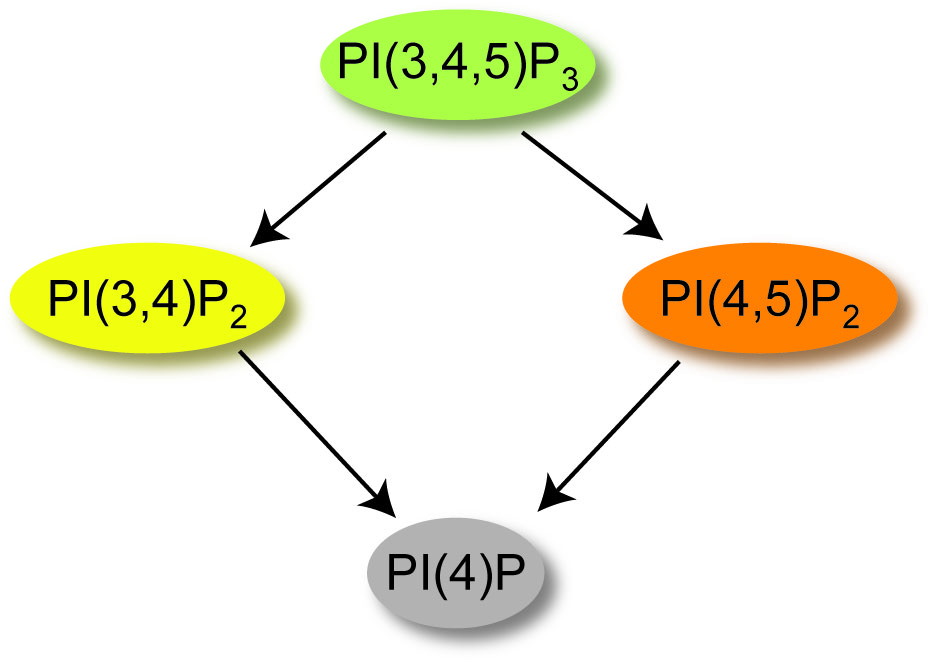
Figure 1. Reactions catalyzed by VSP
There are two main arms of the lab, investigating the biophysical properties of VSP and investigating the physiological function of VSP.
Biophysical Projects
We study how VSP converts an electrical signal into a chemical signal. Our past work looked at how all the different parts of VSP work together. VSP is made up of a voltage sensing domain (VSD), a phosphatase domain and a linker loop that connects the two. Interestingly, the phosphatase domain contains two subdomains, a catalytic domain and a C2 domain. Each domain appears to have its own function.
VSDs are made up of four transmembrane helixes, labeled S1-S4. The charge carrying arginines responsible for sensing voltage are found on the S4 (Figure 1). Our research has shown that the VSD changes conformation during depolarizations to turn on the enzyme (1). The conformational changes are similar to those observed from VSDs on ion channels (2). This means that the vast amount of research on ion channel VSDs likely applies to VSP as well.
Physiology Projects
While our work and the work of others have made significant progress on understanding how VSP works on a biophysical level, much is still unknown regarding the physiological function of VSP. Many phosphatases were already known that can dephosphorylate either the 3- or the 5-phosphates from PIPs. Why does the cell require another one that is regulated by voltage?