Research
Research
Research
The sense of taste is perceived by lingual sensory organ, tongue. The tongue comes in contact with a myriad of consumables and orchestrate decisions to reject or ingest. Mammalian tongue comprises several taste organs called papillae and have resident taste buds (TB). In addition, large area of tongue is covered by non-taste organ that have roles in eating and manipulating the food bolus. The anterior tongue includes a patterned array of taste fungiform papillae (FP) surrounded by non-taste filiform papillae (FILIF). The posterior tongue includes a single circumvallate (CV) papilla in rodent and unlike FP, includes 100s of TB. The taste bud is further heterogeneous and include different cell types to recognize the 5 taste stimulus, salt, sour, sweet, bitter and umami. The tongue and taste organs are innervated from three cranial ganglia: the trigeminal, geniculate, and petrosal. The trigeminal ganglion (Vth) innervates the anterior tongue, FP, and FILIF via the lingual nerve. The geniculate ganglion (VIIth) via chorda tympani provides innervation only to TB in the anterior tongue. The petrosal ganglion (IXth) neurons innervate TB on the posterior tongue, via the glossopharyngeal nerve.
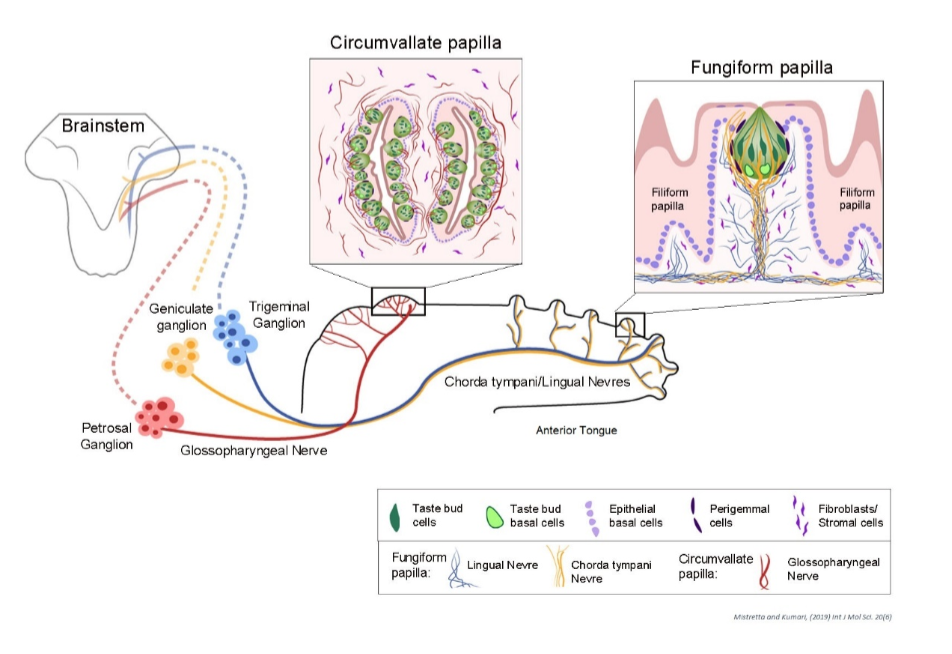
Maintenance of the specialized taste papillae/TB structures is controlled by signaling networks within the tongue epithelium, and between the epithelium and nerves or underlying connective tissue core. Hedgehog (HH) signaling is well established in embryonic tongue development and in maintaining adult taste fungiform papillae, but its role in the maturation of non-taste structures—such as filiform papillae, stromal tissues, and muscles—remains unknown. I hypothesize that HH signaling, mediated by the receptors PTCH1, CDON, and GAS1, is essential for the coordinated maturation of these tissues.
We use transgenic mouse models and perform detailed morphological, histological, and molecular analyses to define how HH signaling shapes postnatal tongue and taste organ architecture. We use fluorescence-activated cell sorting to enable transcriptomic profiling and identification of HH-regulated genes across distinct microenvironments. These studies will uncover the cellular and molecular mechanisms underlying postnatal tongue growth and provide a foundation for investigating HH receptor function in pediatric tongue disorders, with the long-term goal of informing therapeutic strategies.
In parallel with my basic science investigations, I have developed a translational research program evaluating HH pathway inhibition as a potential therapeutic strategy for Oral Squamous Cell Carcinoma (OSCC). In collaboration with Dr. Gary Goldberg (Cell and Molecular Biology, SOM), I conducted pilot studies using patient-derived OSCC cells to test the efficacy of the HH pathway inhibitor sonidegib. These initial findings demonstrated that HH pathway blockade significantly reduces OSCC cell growth and viability, providing a strong foundation for therapeutic exploration.