Research
Research
Our Research
The Tice Lab investigates how chronic conditions like HIV infection and repetitive head trauma disrupt brain homeostasis, contributing to cognitive decline and neurodegeneration. Our work focuses on identifying molecular mechanisms that impair waste clearance, alter astrocyte function, and compromise synaptic stability. By integrating cellular models, human tissue studies, and high-throughput omics techniques, we aim to uncover convergent pathways across neurologic disease states and translate our findings into therapeutic strategies.
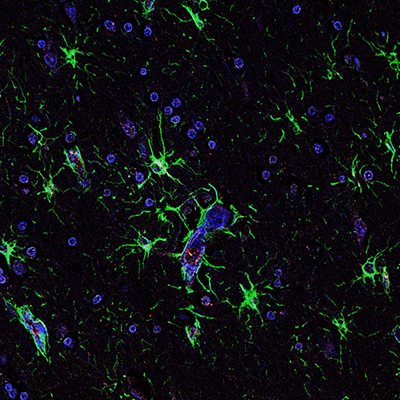
HIV and Glymphatic Dysfunction
One major focus of the lab is understanding how HIV infection alters brain water homeostasis and protein clearance by disrupting the function and localization of aquaporin-4 (AQP4) water channels on astrocyte end feet. These channels are critical for glymphatic exchange, a process that removes toxic proteins and metabolic waste from the brain. Our studies have shown that HIV triggers AQP4 mislocalization through purinergic signaling, lipid raft remodeling, and cytoskeletal transport dysregulation. In collaboration with Dr. Dianne Langford’s lab, we use phospho-mutant AQP4 constructs, human neurovascular co-culture models, and aging EcoHIV mouse models to dissect the mechanisms underlying these changes. Our goal is to identify the cellular processes and therapeutic targets that can protect cognitive function in people living with HIV, particularly as they age.
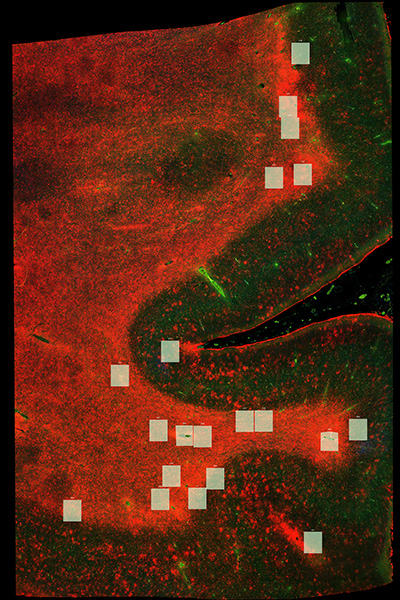
CTE, Parkinson’s Disease, and Shared Molecular Pathways
The lab also investigates how chronic traumatic encephalopathy (CTE) and Parkinson’s disease may converge at the molecular level. Through large-scale transcriptomic and proteomic analyses, we are identifying overlapping changes in mitochondrial function, synaptic regulation, and inflammatory signaling. In collaboration with the Langford Lab, we are especially interested in understanding whether specific cell types, such as parvalbumin-positive interneurons, are particularly vulnerable across trauma-related and degenerative brain diseases. By combining spatial proteomics, single-cell analysis, and disease meta-analyses, our work seeks to define shared biological failure points that may serve as early biomarkers or intervention targets for neuropsychiatric and neurodegenerative conditions.
