Research
Research
Research
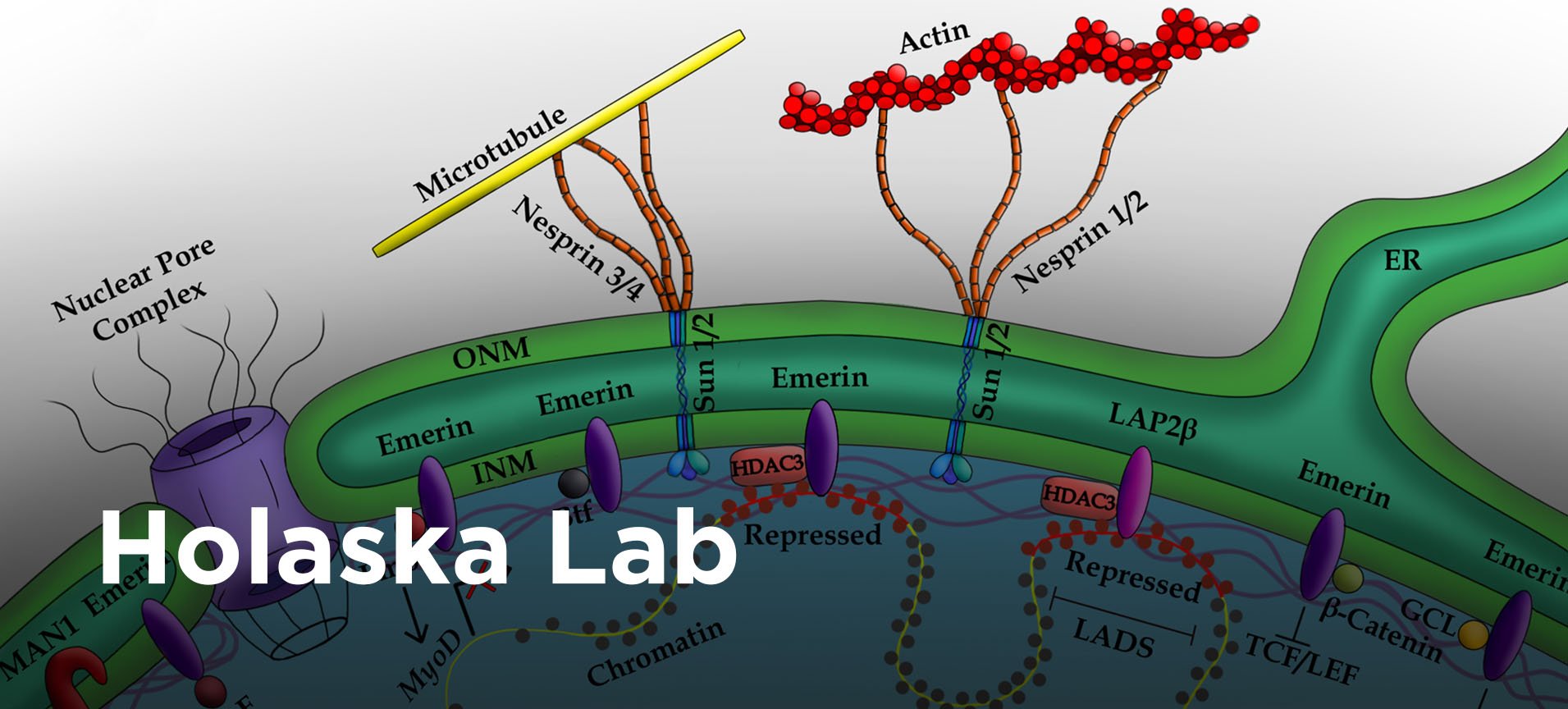
The focus of my research program is on unraveling the molecular mechanisms underlying how the nuclear envelope regulates fundamental cellular processes, including genomic architecture, RNA transcription, cell signaling and maintenance of nuclear structure. The field lacks a fundamental understanding of the mechanisms underlying how proteins at the nuclear envelope regulate transcription factor activity, genomic organization and nuclear architecture. My lab studies an inner nuclear envelope protein named emerin to elucidate these mechanisms.
Mutations in emerin cause Emery-Dreifuss muscular dystrophy (EDMD), a disorder characterized by skeletal muscle wasting and irregular heart rhythms. Recent evidence suggests impaired skeletal muscle stem cell differentiation during healing contributes to the skeletal muscle phenotype of EDMD. Thus, understanding emerin function in nuclear architecture, gene expression and cell signaling during skeletal muscle stem cell differentiation will not only answer fundamental questions regarding how the nuclear envelope regulates genomic architecture and gene expression, but it was also contribute significantly to elucidating the EDMD mechanism.
Studying emerin structure and function will also answer fundamental questions regarding how the nuclear envelope regulates nuclear architecture. These mechanisms have important implications for nuclear size and structure as it relates to the normal function of migratory cells in the body, such as immune surveillance cells (e.g., neutrophils) and the abnormal invasive and migratory phenotype of metastatic cancer cells.
Our long-term goal is to define the role of emerin in regulating tumor formation, tumor growth and cancer cell invasion. Current work in the lab aims to define the mechanism underlying how emerin regulation of nuclear architecture modulates nuclear size and stiffness during cancer cell invasion and metastasis. Invasive cancer cells have reduced emerin expression. This led us to propose reduced emerin expression or altered emerin function is a common mechanism used by solid tumors to select for cells with more compliant nuclei that are able to more easily enter and exit the vasculature to colonize distant organs. Thus, understanding how emerin regulates nuclear size and structure during cancer cell transformation will likely lead to novel approaches for blocking cancer metastasis.
Major Areas of Research
My research program consists of four major areas of research: 1) emerin regulation of nuclear structure during metastasis, 2) emerin regulation of genomic architecture, 3) identification and characterization of signaling and transcriptional pathways disrupted in EDMD, and 4) emerin regulation of transcription factor activity.
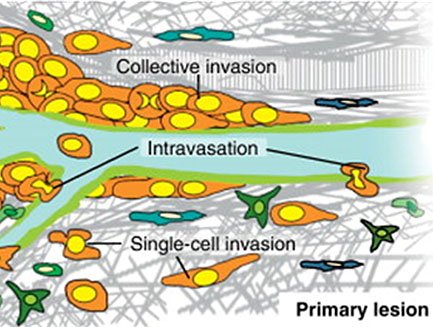
Emerin Regulation of Nuclear Structure During Metastasis
Metastasis accounts for the majority of breast cancer deaths. Metastasis is the spread of cancer from the site of the original tumor to another site in the body. Metastasis requires cancer cells to enter and exit lymphatic and blood vessels...
Emerin Regulation of Genomic Architecture
The field lacks a fundamental understanding of the mechanisms underlying how proteins at the nuclear envelope regulate transcription factor activity and genomic organization. Further, our understanding of how genomic organization at the nuclear periphery is initiated and maintained to...
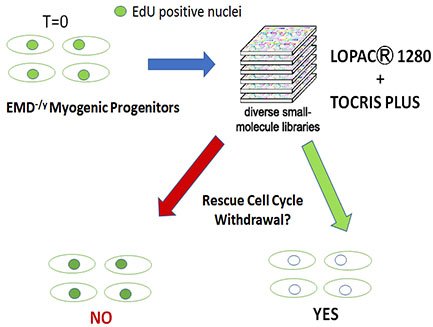
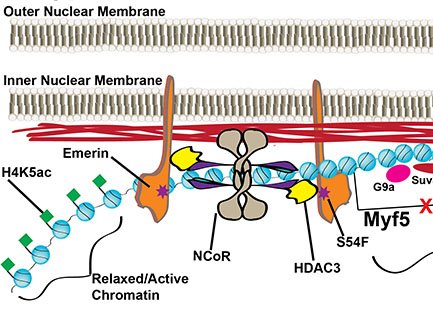
Identification and Characterization of Signaling and Transcriptional Pathways Disrupted in EDMD
We showed emerin-null myogenic progenitors fail to transcriptionally reprogram upon differentiation induction, which we predict is caused by the inability of emerin-null cells to reorganize the genome. Supporting this hypothesis...
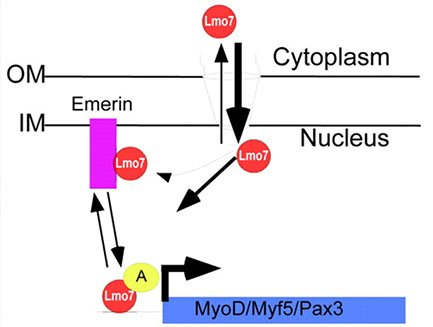
Emerin Regulation of Transcription Factory Activity
We were the first to show emerin binds to many transcription regulators and inhibits their activity. We focus on one of these transcription regulators, Lim-domain only 7 (Lmo7) because of its apparent role in muscle...