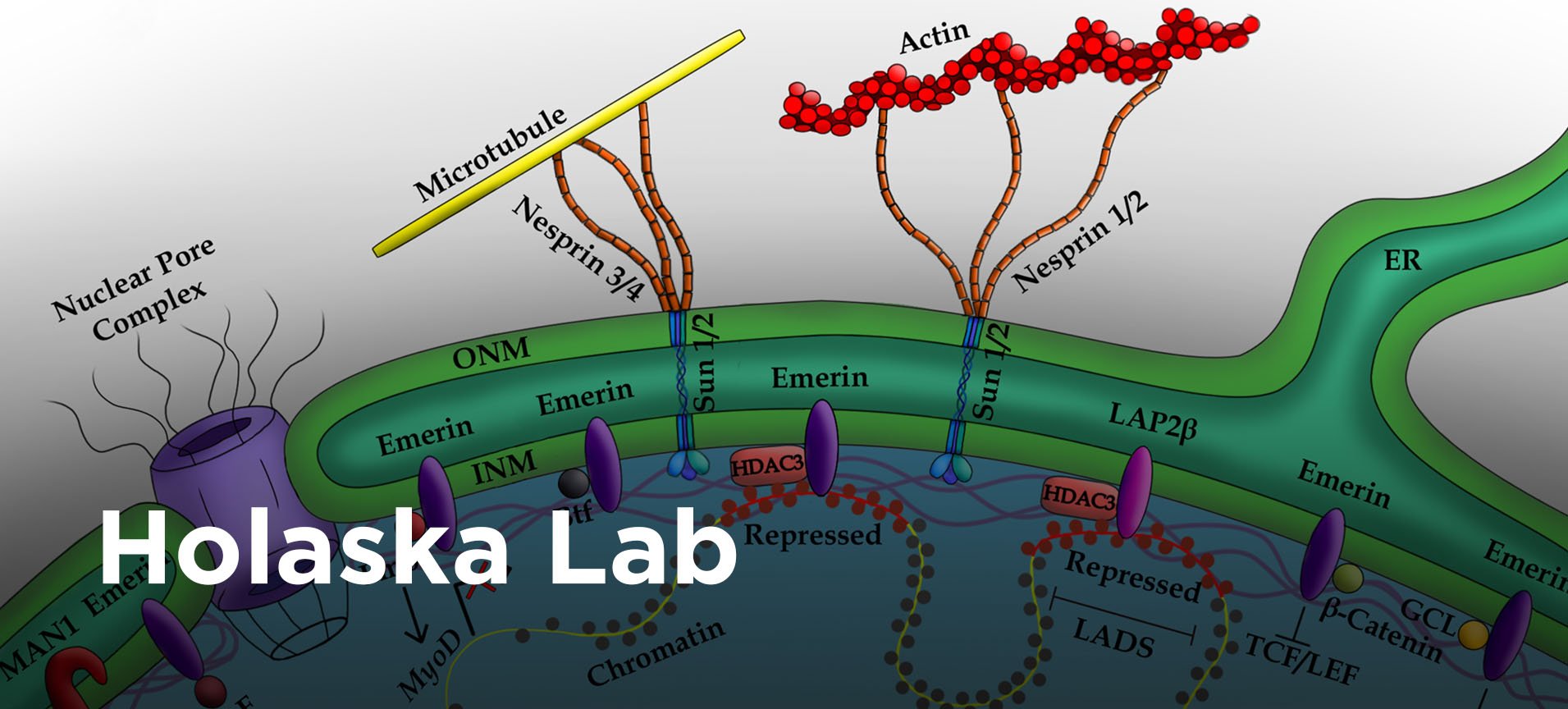
Part I
Part I
Emerin Regulation of Nuclear Structure During Metastatic Transformation
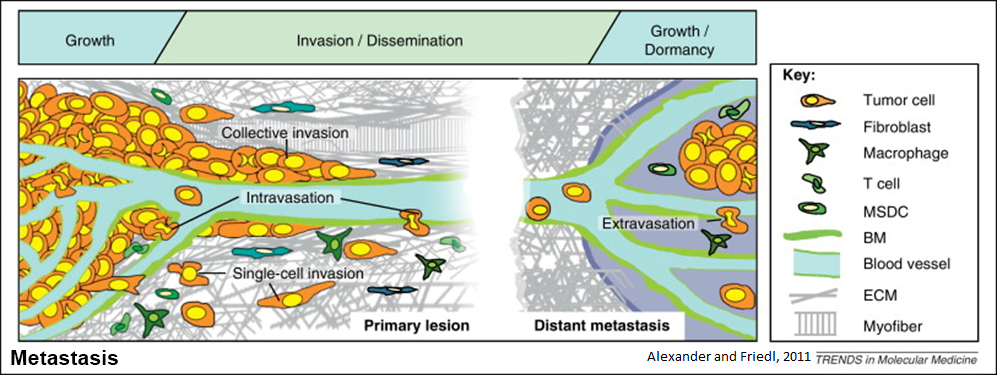
Metastasis accounts for the majority of breast cancer deaths. Metastasis is the spread of cancer from the site of the original tumor to another site in the body. Metastasis requires cancer cells to enter and exit lymphatic and blood vessels through incredibly small gaps; without this, metastatic tumors cannot form. The nucleus is the largest organelle with a diameter of approximately 10-15 µm. The nucleus is the major physical barrier to cells migrating through these narrow gaps, which are often 10% of the nuclear diameter.
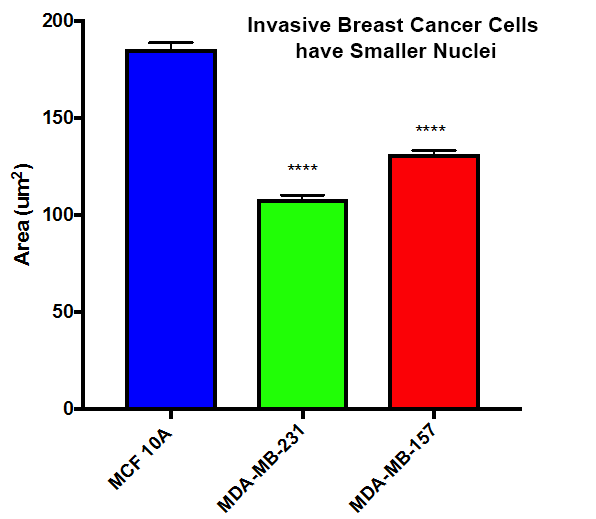
The nucleus is very rigid in normal cells and resists the compressive forces required to contort the nuclei to fit through these gaps. Invasive cancer cells often have smaller, misshapen nuclei, suggesting these changes in nuclear size and structure allow these cells to squeeze through the narrow gaps during metastasis.
How do nuclear size and shape change in cancer cells? Growing evidence suggests nuclear envelope proteins are important regulators of nuclear size and structural changes. Some insights were provided by studying neutrophils, whose function requires them to squeeze through gaps in the capillary endothelium. Neutrophil nuclei are highly compliant and show significantly reduced expression of nuclear envelope proteins, including emerin. Altered expression of nuclear lamina proteins occurs in many cancers and often correlates with negative clinical outcomes.
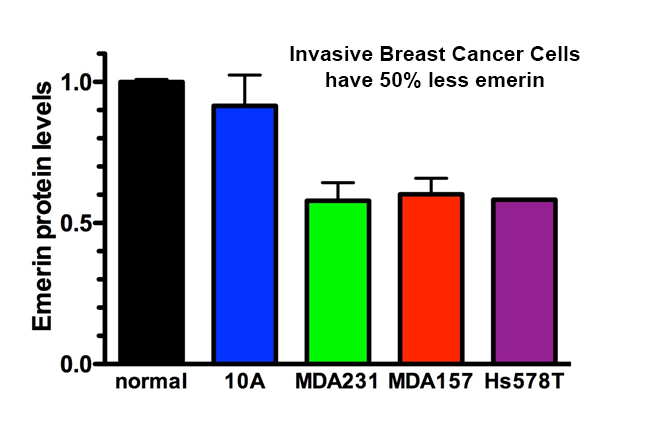
The invasive breast cancer cell line MDA-MB-231 has altered expression of nuclear envelope proteins, including emerin, and exhibits greater nuclear deformation and faster migration through tight spaces.Emerin binds to many structural proteins in the nucleus, including nuclear actin, nuclear myosin I (NMI), nuclear protein 4.1R and aII-spectrin. Short, nuclear actin filaments stabilized by emerin at the nuclear envelope provide added support to the nuclear envelope. These interactions are predicted to form a stable nucleoskeleton containing lamin filaments and actin filaments physically linked via emerin. Collectively, these findings support a prominent role for emerin in regulating nuclear structure during cancer transformation.
Our recent evidence has begun to elucidate the mechanism(s) by which emerin regulates nuclear size and structure to control migration through small pores in vitro and in metastasis.
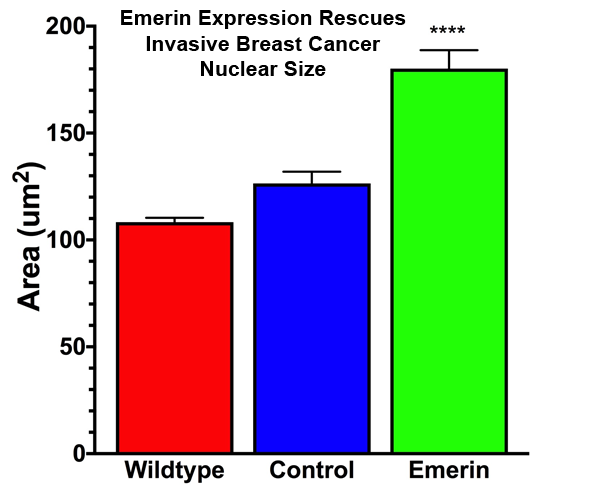
Elucidating the molecular mechanisms by which nuclear envelope proteins regulate nuclear size and structure to affect cancer cell invasion will contribute to our understanding of cancer cell transformation and metastasis. It is imperative that we gain a better understanding of this processbecause inhibiting these nuclear structural changes are predicted to impair cell squeezing through endothelial slits and thus block metastasis. Thus, these studies will identify new therapeutic targets for tumor metastasis and has the potential to impact cancer survival.
| Part II ⇒ |